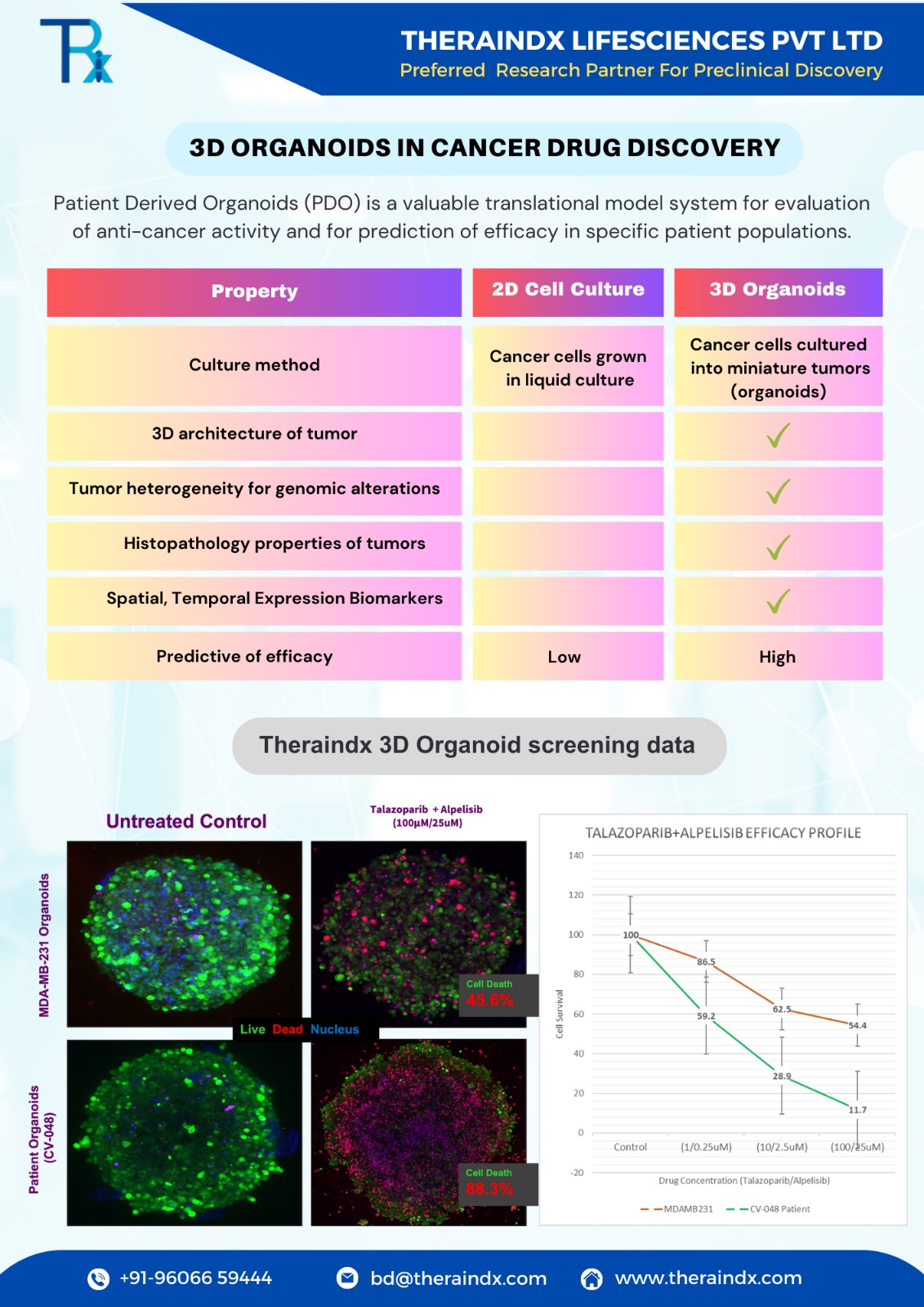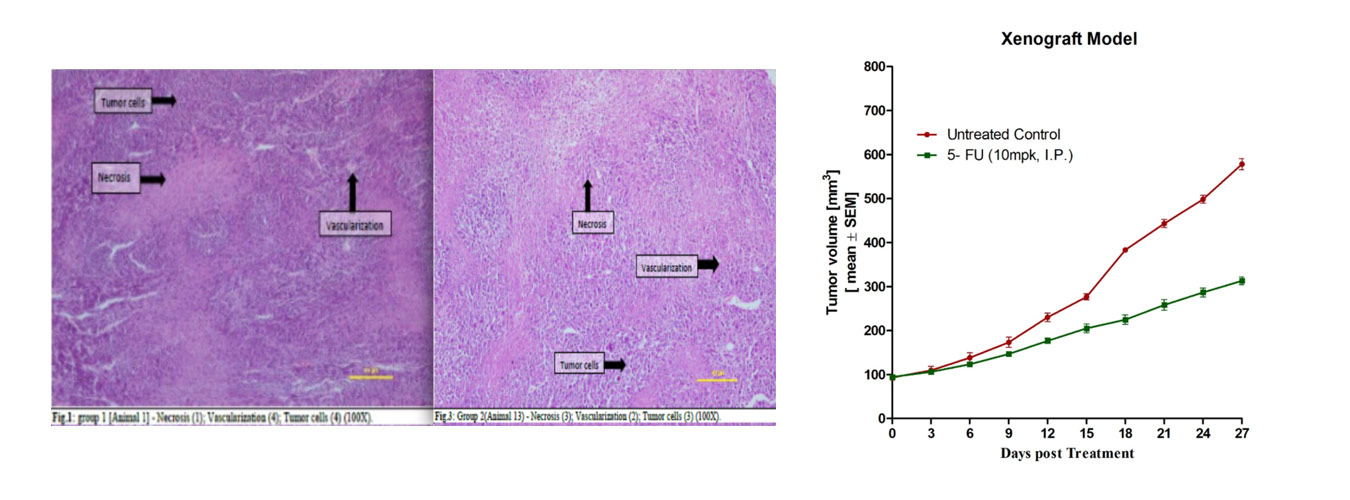
Cancer is one of the most difficult disease to treat with high mortality rates of 92.3% within five years of diagnosis. A major challenge encountered in cancer drug discovery and development is high attrition rates of development candidates in clinical trials due to lack of efficacy. This lack of efficacy in clinical studies has been attributed to low accuracy of preclinical models to predict efficacy of compounds in the clinic. As a result, preclinical models with high predictive value for efficacy are urgently required.
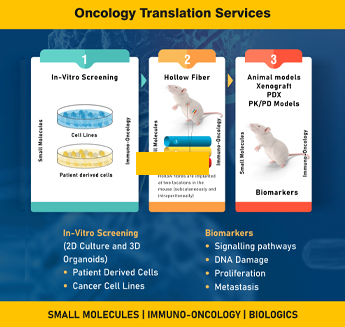
Discover TheraIndx Oncology, where ground breaking cancer drug discovery and development thrives through advanced preclinical models and unparalleled expertise. Our experienced Oncology team offers a comprehensive range of innovative solutions, including 2D/3D Organoid models, Hollow Fiber models, iPSC screening platform, as well as PDX and GEM models. These models are meticulously designed to replicate and explore intricate tumor environments with precision. Integrated pharmacology models further enhance our capabilities by incorporating biomarkers, ensuring robust translational insights from preclinical stages to clinical development. At TheraIndx, we specialize in bridging the gap between discovery and application, providing tailored approaches and comprehensive support to accelerate your oncology research.