Identification of GDC-1971 (RLY-1971), a SHP2 Inhibitor Designed for the Treatment of Solid Tumors
The non-receptor protein tyrosine phosphatase SHP2 (Src Homology Phosphatase-2), encoded by the PTPN11 gene, plays an important role in the transduction of signalling in the cell. SHP2 has been shown to regulate multiple signalling cascades, including the RAS/RAF, PI3K/AKT, JAK/STAT pathways etc. Mutations and/or the overexpression of SHP2 has been associated with genetic diseases as well as various cancers. Because of its role in regulating signalling cascades, therapeutic approaches where SHP2 inhibition is combined with an inhibitor of RAS or certain receptor tyrosine kinases (RTKs) are of increasing interest.
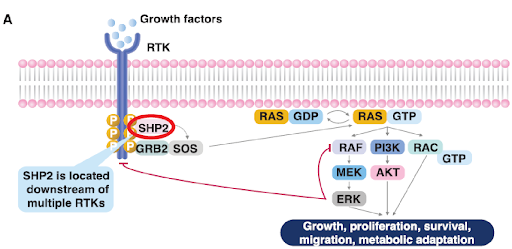
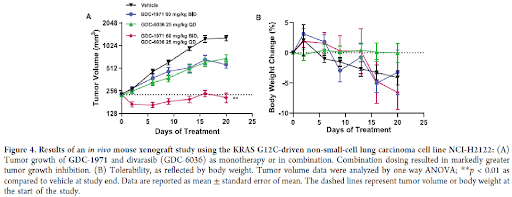
Identification and characterization of GDC-1971, a SHP2 inhibitor currently in clinical trials in combination with KRASG12C inhibitor, Divarasib (GDC-6036) for the treatment of solid tumors, was described. To test whether SHP2 inhibition together with KRAS inhibition could result in potent antitumor activity, GDC-1971 was tested in combination with the potent and selective KRASG12C inhibitor, Divarasib, in vivo.
The KRASG12C mutant non-small cell lung carcinoma (NSCLC) xenograft model NCI-H2122 was established in nude mice, and tumor-bearing animals were treated with GDC-1971 (60 mg/kg, BID) or divarasib (25 mg/kg QD) as single agents or in combination. These doses were below the maximum tolerated doses for both compounds. Compounds were administered orally for 21 days, and the tumor volume and animal weight were monitored over the course of the treatment. In the single agent cohorts, a modest reduction in tumor growth was observed. However, the combination of GDC-1971 and Divarasib resulted in a significant reduction in tumor growth. The compounds were well tolerated, with no significant loss of body weight. Taken together, these in vivo efficacy data supported the potential for GDC-1971 both in monotherapy and in combination with RTK- and RAS/MAPK-driven cancers.
Synthesis of GDC-1971 was achieved using the following 3 schemes thereby installing the central core, the chiral amine part and the final synthesis. After the initial coupling of intermediate 45 (from Scheme-1) with tetrahydronaphthyridine bicyclic ring using Pd and Xantphos, the resultant heteroaryl chloride 54 underwent nucleophilic aromatic substitution with the bis-HCl salt 53 (from Scheme-2) in DMSO and K2CO3 to provide the tetrahydropyran-protected analogue 55, that was deprotected using HCl in MeOH to furnish the desired compound GDC-1971 as HCl salt.
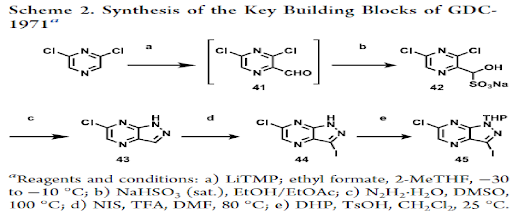
Scheme 1: Synthesis of the central core (Intermediate-45) of GDC-1971
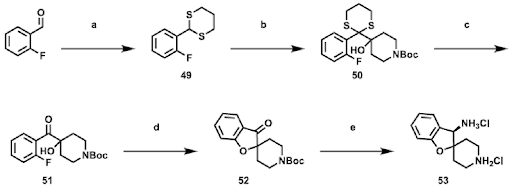
Scheme 2: Synthesis of the chiral amine salt (Intermediate-53) of GDC-1971
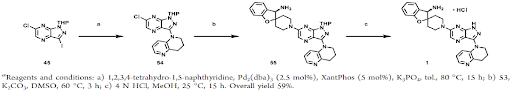
Scheme 2: Synthesis of the chiral amine salt (Intermediate-53) of GDC-1971
- 1. Alexander M. Taylor et al. Identification of GDC-1971 (RLY-1971), a SHP2 Inhibitor Designed for the Treatment of Solid Tumors. J. Med. Chem. 2023, 66, 13384−13399.
- 2. Asmamaw, M. D. et al. A comprehensive review of SHP2 and its role in cancer. Cell Oncol. 2022, 45 (5), 729−753.
- 3. Shen, D. et al. Therapeutic potential of targeting SHP2 in human developmental disorders and cancers. Eur. J. Med. Chem. 2020, 190, 112−117.
- Author : Dr. Sachin Madan – Director Medicinal Chemistry at TheraIndx Lifesciences Pvt ltd




