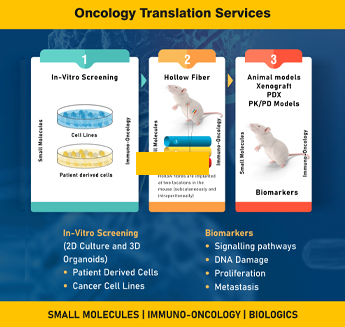
Subcutaneous Xenograft Models
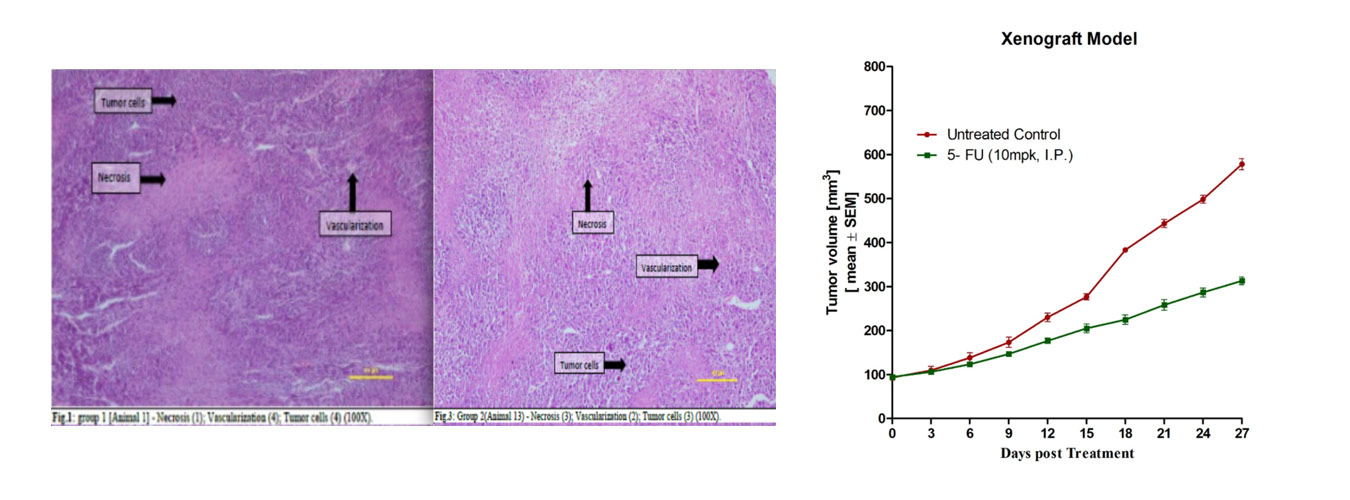
Subcutaneous Xenograft models involve the implantation of human cancer cells or patient-derived tumor tissues directly under the skin of immunodeficient animals. This model allows for straightforward monitoring of tumor growth, evaluation of treatment responses, and assessment of drug efficacy.

Orthotopic Xenograft Models
Orthotopic Xenograft models involve the transplantation of human cancer cells or tumor tissues into the anatomically relevant site in animals, mimicking the original location of the tumor in humans. This model allows researchers to study tumor invasion, metastasis, and the interactions between tumors and surrounding tissues.

Metastatic Xenograft Models
Metastatic Xenograft models involve the introduction of human cancer cells into animals to study the process of metastasis, the spread of cancer cells from the primary tumor to distant organs. These models are instrumental in understanding the mechanisms of metastasis, evaluating potential antimetastatic therapies, and developing strategies to prevent or treat metastatic disease.

Patient-Derived Xenograft (PDX) Models
Patient-derived Xenograft (PDX) models involve the transplantation of patient-derived tumor tissues directly into immunodeficient animals. This model retains the genetic and histological characteristics of the original tumor, enabling personalized medicine approaches. PDX models allow researchers to evaluate individual patient responses to various treatment modalities, facilitating the development of tailored therapies and enhancing clinical decision-making.

Xenograft Models for Hematological Malignancies
Xenograft models are widely used for studying hematological malignancies, including leukemia, lymphoma, and multiple myeloma. These models involve the transplantation of human hematopoietic cancer cells or patient-derived samples into immunodeficient animals, enabling the evaluation of disease progression, therapeutic responses, and the development of novel treatment strategies.

Humanized Xenograft Models
Humanized Xenograft models involve the engraftment of human immune cells, such as hematopoietic stem cells or immune cell subsets, into immunodeficient animals. These models allow researchers to study the interactions between human tumors and the human immune system, investigate immune responses to cancer, and evaluate the efficacy of immunotherapies.

Xenograft Models for Drug Resistance Studies
Xenograft models are widely used to study mechanisms of drug resistance in cancer. Researchers can establish Xenograft models using tumor cells or tissues that have acquired resistance to specific therapies. These models help elucidate the genetic, molecular, and microenvironmental factors driving resistance, facilitating the development of strategies to overcome resistance and improve treatment outcomes.