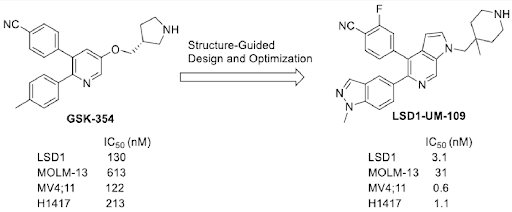
Pyrrolo[2,3‑c]pyridines as Potent and Reversible LSD1 Inhibitors
Lysine specific demethylase 1 (LSD1) is a promising therapeutic target for the treatment of cancer, and a number of LSD1 inhibitors had advanced into clinical development. It acts as an epigenetic eraser by specifically demethylating histone 3-lysine 4 (H3K4) and histone 3- lysine 9 (H3K9) residues. A series of pyrrolo[2,3-c]pyridines as a new class of highly potent and reversible LSD1 inhibitors were designed based on previously reported LSD1 inhibitor GSK-354. Among them, lead compound 46 (LSD1-UM-109) showed an IC 50 value of 3.1 nM in LSD1 enzymatic activity as compared to 130 nM (GSK-354) and inhibited cell growth in MV4-11 & MOLM-13, both acute myeloid leukemia (AML) cell lines and also H1417, small cell lung cancer (SCLC) cell line.
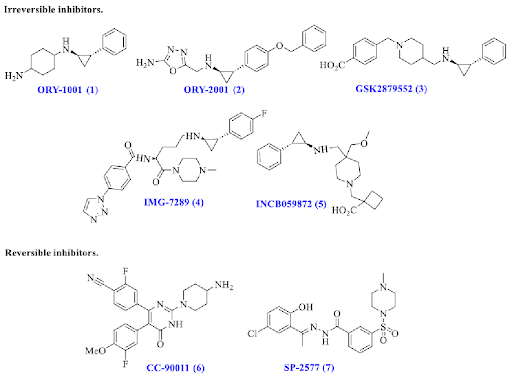
In the past decade, extensive research efforts have been directed to the discovery of LSD1 inhibitors, which are broadly divided into 2 classes namely, reversible and irreversible inhibitors based upon their mode of action. To date, five irreversible and two such reversible inhibitors have advanced to the human clinical trials.
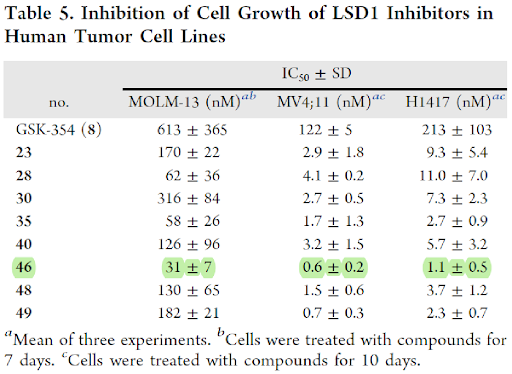
Although cell growth in three cell lines, MOLM-13, MV4-11 (AML) and H1417 (SCLC) was inhibited by a number of LSD1 inhibitors that were more potent than GSK-354, however in direct comparison, the lead compound 46 (IC50 values of 0.6 nM in MV4-11; 31 nM in MOLM-13 and 1.1 nM in H1417) was 20-, 201-, and 193-times more potent than GSK-354. Also, compound 49 achieved IC50 values of 182 nM, 0.7 nM, 2.3 nM in inhibition of cell growth in MOLM-14, MV4-11 and H1417 cell lines, respectively, and was more potent than GSK-354.
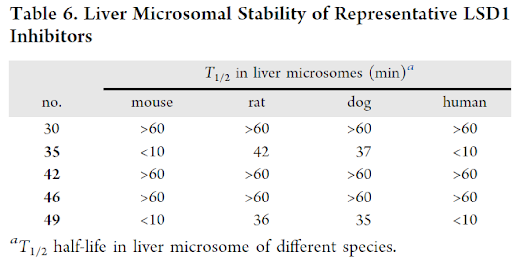
Next, these compounds were evaluated for liver microsome stability. Compounds 30, 42 and 46 showed excellent microsomal stability in mouse, rat, dog and human liver microsomes. However, compounds 35 and 49 had moderate stability for rat & dog and very poor stability in mouse and human liver microsomes.
- 1. Shaomeng Wang et. al. Discovery of Pyrrolo[2,3‑c]pyridines as Potent and Reversible LSD1 Inhibitors. ACS Med. Chem. Lett. 2023 14 (10), 1389-1395.
- 2. Fu, X. et al. Advances toward LSD1 inhibitors for cancer therapy. Future Med. Chem. 2017, 9 (11), 1227−1242.
- 3. Amente, S. et. al. The histone LSD1 demethylase in stemness and cancer transcription programs. Biochim. Biophys. Acta 2013, 1829 (10), 981−986.
- Author : Dr. Sachin Madan – Director Medicinal Chemistry




