Conducting Intracutaneous Reactivity Tests for Medical Devices: A Guide to ISO 10993-23 Compliance
Introduction :
The Intracutaneous Reactivity Test is a part of the biological evaluation of medical devices, as outlined in ISO 10993-23. This test provides guidance on conducting this specific test. The Intracutaneous Reactivity Test is designed to assess the potential of a medical device or its extract to induce irritation or sensitization when implanted into the subcutaneous tissue. Here are the general considerations and steps for conducting the Intracutaneous Reactivity Test in accordance with ISO 10993-23:
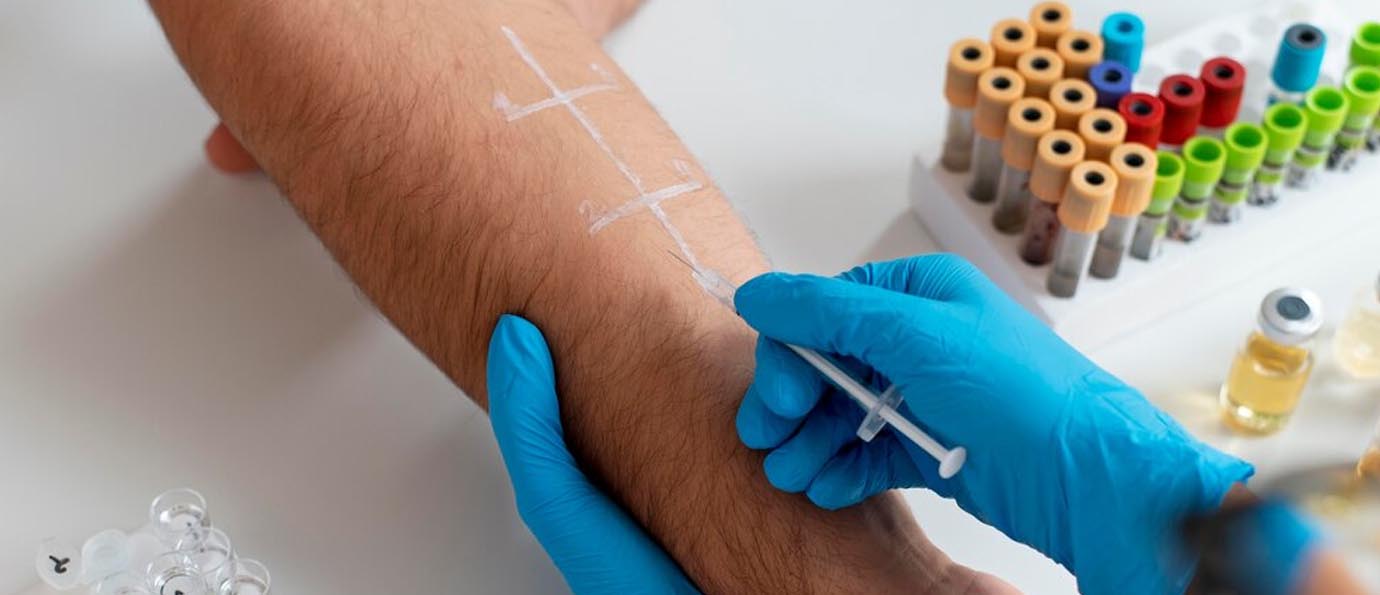
Understanding ISO 10993-23:
ISO 10993-23:2021 is the latest guidance that focuses specifically on irritation testing. This part of the ISO 10993 series represents a significant update, emphasizing a more refined approach to evaluating irritation potential, including both in vivo (using live animals) and in vitro (using cell cultures or tissue models) methods. The standard underscores the need to consider ethical aspects of testing, advocating for the reduction, refinement, and replacement (3Rs) of animal testing wherever possible.
Process of Testing
The process of Intracutaneous Reactivity Testing for medical devices, as outlined by standards such as ISO 10993-23, involves a series of steps designed to assess the potential of a device or its components to cause irritation or sensitization when in contact with skin or implanted into the subcutaneous tissue. Here's a detailed overview of the process:
1. Test Objectives and Scope:
Clearly define the objectives of the Intracutaneous Reactivity Test for the specific medical device in question. Identify the scope of the test, including the nature and duration of the implantation.
2. Selection of Test Animals:
Choose appropriate animal species, such as rabbits, for conducting the test. Ensure that the selected species is relevant to the intended use of the medical device.
3. Selection of Test Sites:
Identify suitable sites for implantation on the test animals. ISO 10993-23 provides guidance on the recommended implantation sites.
4. Implantation of Test Material:
Implant the medical device or its extract into the subcutaneous tissue of the test animals. Follow the guidelines in ISO 10993-23 for the appropriate implantation procedure.
5. Monitoring and Observation:
Monitor the test sites regularly for signs of irritation or other adverse reactions. Document any observable changes, such as erythema, edema, or necrosis.
6. Duration of Implantation:
Determine the appropriate duration for implantation based on the characteristics of the medical device and its intended use. ISO 10993-23 provides recommendations on implantation periods.
7. Histopathological Evaluation:
At the end of the implantation period, perform a histopathological evaluation of the implantation sites. Assess the tissue reactions and any inflammatory responses.
8. Data Analysis:
Analyze the data collected during the study, including observations and histopathological findings. Evaluate the intracutaneous reactivity of the medical device or its extract.
9. Reporting:
Prepare a comprehensive report summarizing the experimental design, test conditions, observations, histopathological findings, and conclusions. Include any relevant information on the medical device, its composition, and processing conditions.
10. Quality Assurance and Compliance:
Ensure that the testing is conducted in compliance with ISO 10993-23 and any additional regulatory requirements. Implement quality assurance measures to maintain the integrity and reliability of the test results.
Conclusion
It's crucial to note that ISO 10993-23 provides general guidelines for conducting the Intracutaneous Reactivity Test, and specific details may vary based on the nature of the medical device and its intended use. Laboratories conducting these tests should have expertise in biological evaluation and follow relevant guidelines to ensure accurate and reliable results. Staying abreast of the latest scientific advancements in testing methodologies is essential for maintaining compliance and upholding the highest standards of safety evaluation. Collaboration with regulatory bodies and participation in professional forums dedicated to medical device testing can further enhance the quality and relevance of the biological evaluation process.




